Tolomatic Develops Ventilator Actuation Prototypes, Seeks Partner for Final Design
Minneapolis company Tolomatic has developed a new type of manual resuscitator using an electric linear actuator, according to an April 13 press release.
The prototypes of the new device automate a non-invasive, positive-pressure resuscitator, known as a bag valve mask, or artificial manual breathing unit (AMBU). The device, used primarily in emergency situations when traditional ventilators are not available, delivers oxygen to patients requiring breathing assistance. The bag valve mask is positioned over the patient’s nose and mouth. A medical technician manually squeezes the bag to provide a combination of ambient air and oxygen. Squeezing the bag manually is workable for short durations but not viable for longer-term care. It can also create air flow inconsistencies, as well as require extra time and labor to stabilize the patient.
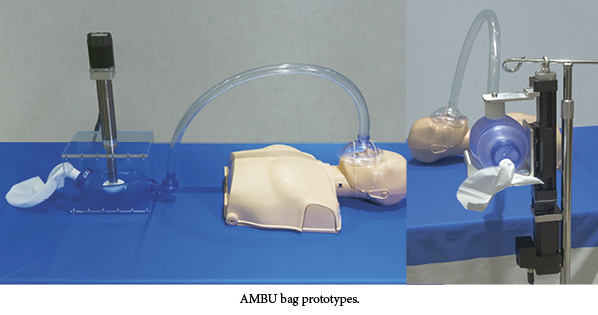
Tolomatic’s approach is to automate this traditionally manual process using their electric linear actuators and ensure that patients continue to receive air for days or weeks. The company said it hopes to spark interest and conversation with potential partners in developing a final design solution that can be submitted for approval, according to the press release.
Tolomatic’s screw-driven linear actuators convert rotary power from a servo motor into linear motion, providing smooth and consistent operation of the system and allowing the device to control the velocity, acceleration and distance of any move at any point in time. The controlled motion allows for a more continuous volume of air per compression cycle and a more typical breathing cycle.
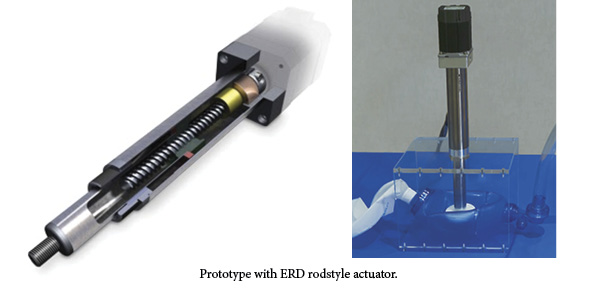
AMBU bags with an automated single-direction camming solution or pneumatic actuator do not allow for varying the stroke length, only the breath frequency. As a result, the tidal volume of air flow to the patient is always the same, and the motion profile is counter to a typical breath cycle.
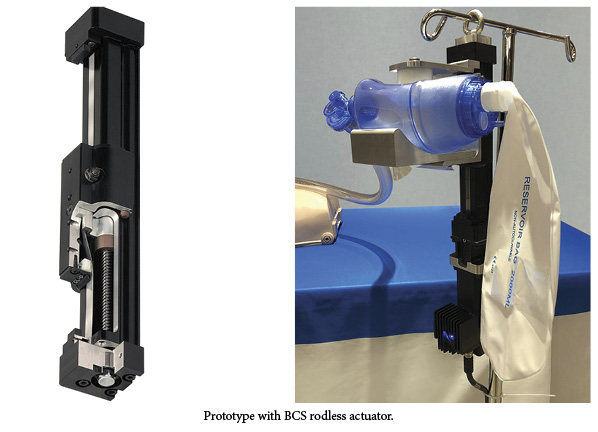
In contrast, linear actuator systems can change the frequency of the induced respirations and also the volume, which is not possible with fixed-displacement rotary devices. The total control of motion from a linear electric actuator allows for much more flexible air flow that could now be modified for a patient’s age, size or current needs.
Tolomatic is seeking partners for a final design of the prototype. The new type of respirator could be used to treat patients with COVID-19, a respiratory illness.
“We are not a medical-device manufacturer, and this design has not been approved by any regulatory bodies,” Andy Zaske, Tolomatic vice president of sales and marketing said in the press release. “However, many of our customers are, and our hope is that this might spark some interest in partnering with us on an approved final design solution. We stand ready to offer our motion control expertise to help solve critical challenges during this time.”
For more information, contact Tolomatic, 3800 County Road 116, Minneapolis, MN 55340, call 763-478-8000 or 800-328-2174, or visit www.tolomatic.com.







