Vacuum Measurement: A Basic Guide
 There is often much confusion with regards to the units used to measure the level of vacuum being generated in an industrial application. This article explains which ones are the most common, their origins, when one should be used instead of another, and how to convert between them.
There is often much confusion with regards to the units used to measure the level of vacuum being generated in an industrial application. This article explains which ones are the most common, their origins, when one should be used instead of another, and how to convert between them.
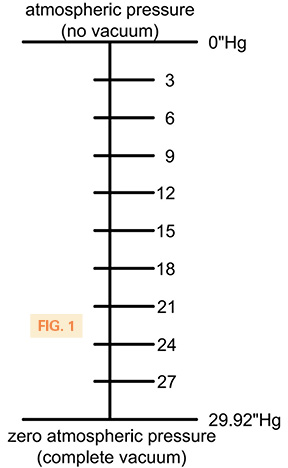
The most common unit of vacuum measurement used in North America for general vacuum is inches of mercury denoted by “Hg, where (“) refers to linear inches and Hg is the chemical symbol for mercury. The most important point to understand about “Hg is that it is a measurement of differential pressure. In vacuum terms, this means that “Hg is the difference between the ambient atmospheric pressure and the vacuum that has been created in an application. Fig. 1 shows this graphically.
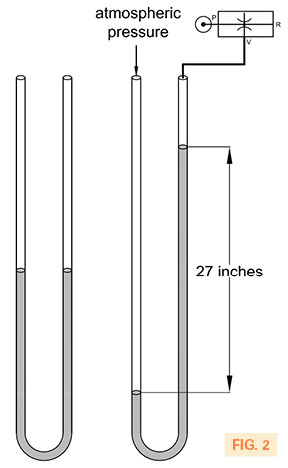
Inches of mercury refers to just that–a linear measurement of mercury. Fig. 2 illustrates a glass tube that is some 3-ft high. Mercury is poured into this tube and settles at the same height on both sides of the apparatus because the atmospheric pressure is the same on each column. When a vacuum is applied to one side of the tube, the higher atmospheric pressure pushes the mercury down, in this case, by 27 inches. Therefore, the vacuum being created is 27″Hg or 27 inches of mercury.
 30″Hg is regarded as the maximum vacuum level available at sea level, and because the oceans are the same height across the planet, this is a good datum point for reference. This number is actually rounded up from 29.92″Hg. 29.92″Hg is the maximum difference in pressure based upon a known atmospheric pressure condition that is internationally agreed upon as 1,013 mbar or 14.7 psi at sea level. This atmospheric pressure continuously changes across the globe. In fact, the highest atmospheric condition ever recorded at sea level was 15.6 psi and the lowest about 12.5 psi, which was taken from within a hurricane. As the atmospheric pressure changes, the maximum differential available changes with it.
30″Hg is regarded as the maximum vacuum level available at sea level, and because the oceans are the same height across the planet, this is a good datum point for reference. This number is actually rounded up from 29.92″Hg. 29.92″Hg is the maximum difference in pressure based upon a known atmospheric pressure condition that is internationally agreed upon as 1,013 mbar or 14.7 psi at sea level. This atmospheric pressure continuously changes across the globe. In fact, the highest atmospheric condition ever recorded at sea level was 15.6 psi and the lowest about 12.5 psi, which was taken from within a hurricane. As the atmospheric pressure changes, the maximum differential available changes with it.
If, for example, the machinery was at a very high altitude, such as in Denver, Co., the atmospheric pressure is reduced and therefore, the possible DIFFERENTIAL pressure that can be created also is reduced. The average atmospheric pressure in Denver is about 12.1 psi, and the pressure differential that can be created is only 24.63″Hg. Therefore, vacuum-lifting apparatus would be less effective by this lower pressure differential or vacuum level.
 Because of this forever-changing atmospheric condition, “Hg should only be used as a guide and in applications where accurate vacuum levels are not required, such as a process-type application. Inches of mercury is ideal for vacuum lifting with vacuum cups, as the amount of vacuum required is rarely high. Typical vacuum handling utilizes anything between 15″Hg and 25″Hg. Therefore, “Hg is suitable as a measurement of system performance in this type of operation.
Because of this forever-changing atmospheric condition, “Hg should only be used as a guide and in applications where accurate vacuum levels are not required, such as a process-type application. Inches of mercury is ideal for vacuum lifting with vacuum cups, as the amount of vacuum required is rarely high. Typical vacuum handling utilizes anything between 15″Hg and 25″Hg. Therefore, “Hg is suitable as a measurement of system performance in this type of operation.
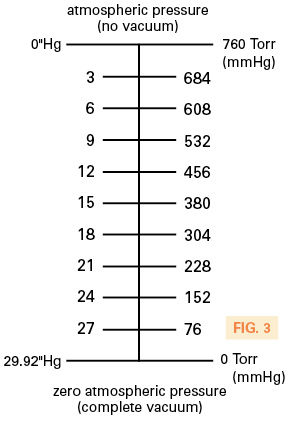
For more accurate vacuum applications where the user must have a known vacuum level, an absolute vacuum unit should be used. Absolute pressure is the relevant factor here, and an absolute measurement takes its reading based on a datum point of zero. Zero is always zero and never changes. In North America, “torr” [a unit of measurement devised by Italian scientist Evangelista Torricelli (b.1608)] is very popular. Torricelli simply measured linear mercury movement using millimeters and based zero on a zero atmospheric condition. Therefore, 29.92 inches converted to millimeters is 760 (759.97). A system running at 50% vacuum is either 380 Torr or 15″Hg. However, the torr measurement scale is more accurate as it has with a datum point of zero atmospheric pressure. 15″Hg is a guide as it’s taken from a varying atmospheric pressure. This conversion is simple using 50%, but if the torr reading were 200, then the equivalent in “Hg would be 21.75. Refer to Fig. 3 for this comparative scale.
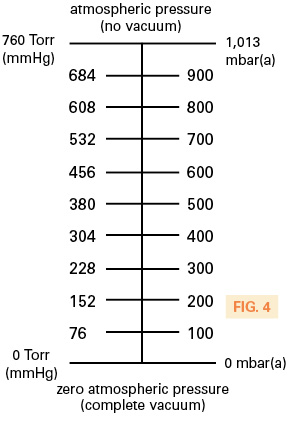 Outside of North America, the mbar(a) unit of vacuum measurement is used. Like torr, this is an absolute pressure scale where 0 is zero atmospheric pressure and 1,013 mbar(a) is the standard for atmospheric pressure. This unit is easily converted to torr simply by multiplying by 0.760. Therefore, 500 mbar equates to 380 Torr. Refer to Fig. 4 for the comparative scale.
Outside of North America, the mbar(a) unit of vacuum measurement is used. Like torr, this is an absolute pressure scale where 0 is zero atmospheric pressure and 1,013 mbar(a) is the standard for atmospheric pressure. This unit is easily converted to torr simply by multiplying by 0.760. Therefore, 500 mbar equates to 380 Torr. Refer to Fig. 4 for the comparative scale.
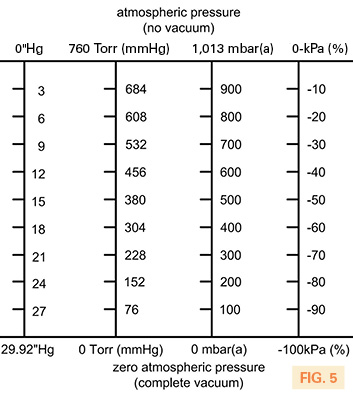
Often used on a vacuum gauge is –kPa. This is a useful unit of measurement as it represents the percentage of vacuum and is widely used when discussing a general vacuum system. The user could, for example, explain to an engineer that they require “about 80% vacuum,” which is -80kPa from atmospheric pressure. Regardless of the location of these two people and what unit of measurement they are more familiar with, percentage vacuum is easy to understand and verbally communicate. Fig. 5 compares all these measurement scales for easy reference.
Vacuum is the removal or reduction of atmospheric pressure. Depending on the application, the vacuum level may require a high accuracy, which means an absolute measurement unit such as torr or mbar(a) should be used, but in general vacuum applications, “Hg offers an easy guide to achieving a basic vacuum condition.
This article is intended as a general guide and as with any industrial application involving machinery choice, independent professional advice should be sought to ensure correct selection and installation.
By Daniel Pascoe, Davasol Inc.
Vacuforce LLC is a manufacturer and distributor of vacuum components and systems for industry in North America. Vacuforce can be reached via its website (www.vacuforce.com) or directly at sales@vacuforce.com. Illustrations and 3D models supplied by Daniel Pascoe at Davasol Inc., an industrial distribution branding company. Daniel can be reached at dpascoe@davasol.com.




fig-1 is incorrect and misleading, because 0″of Hg means complete vacuum and 29″ of Hg means atmospheric pressure. Please correct this information.
pls fix
Mr. Science,
Thank you for your comment. This is an unusual situation where both positions are right, depending on where you put your pressure gauge. The illustration shows the vacuum generator attached to one side of the loop, with the opposite end open to atmosphere. So in this case, the vacuum being measured is in the tube above the mercury. If atmosphere is at standard 14.7 psia, then the vacuum level will be identified as how far up the tube 14.7 psi can push the mercury, which is a maximum of 29.92 “Hg. Zero inches of mercury would mean no vacuum at all, and 29.92 “Hg would be maximum vacuum.
On the other hand, if you are gauging the open end of the looped tube to determine the vacuum level of the environment, then zero inches of mercury would indicate complete vacuum and 29.92 “Hg would signify no vacuum with standard atmospheric pressure.
Figure 1 is consistent with the graphic.
Danyal,
Thank you for your comment. This is an unusual situation where both positions are right, depending on where you put your pressure gauge. The illustration shows the vacuum generator attached to one side of the loop with the opposite end open to atmosphere. So, in this case, the vacuum being measured is in the tube above the mercury. If atmosphere is at standard 14.7 psia, then the vacuum level will be identified as how far up the tube 14.7 psi can push the mercury, which is a maximum of 29.92 “Hg. Zero inches of mercury would mean no vacuum at all and 29.92 “Hg would be maximum vacuum.
On the other hand, if you are gauging the open end of the looped tube to determine the vacuum level of the environment, then zero inches of mercury would indicate complete vacuum and 29.92 “Hg would signify no vacuum with standard atmospheric pressure.
Figure 1 is consistent with the graphic.
So where does the denomination of “microns” of vacuum as used in air conditioning servicing fall in relation to inches of Hg or mb?